Introduction
The Identification of Medicinal Product (IDMP) regulation aims to facilitate the unique identification and exchange of medicinal product and substance information through the establishment of an internationally accepted framework of standards. As the standards of IDMP are implemented, life science companies need to consider how to ensure robust data management strategies are in place to meet compliance requirements. Consequently, it is also an opportunity for companies to understand how to get the most from their data and strategize a well-planned approach that can help turn data into assets.

What Is IDMP?
In 2012, five ISO IDMP standards were released which make up the framework for the IDMP initiative. These standards define an IDMP data model consisting of data elements and their corresponding relationships for information exchange and the identification of medicinal products. They describe in detail all the information needed to uniquely identify an approved drug that is available for sale in jurisdictions that a Health Authority oversees, including the following:
- Medicinal product information
- Pharmaceutical product information
- Substance characteristics
- Dose forms, unit of presentation, routes of administration, and packaging
- Units of measurement
The ISO IDMP standards were updated in 2017 to clarify some of the relationships and data needed in this detailed data model for medicinal products. However, not all regulatory agencies have determined how to implement these standards. Until final decisions are made in terms of implementation, there are many systems and processes that life science companies can use to ensure compliance and to gain a competitive advantage in capturing and maintaining this critical data.
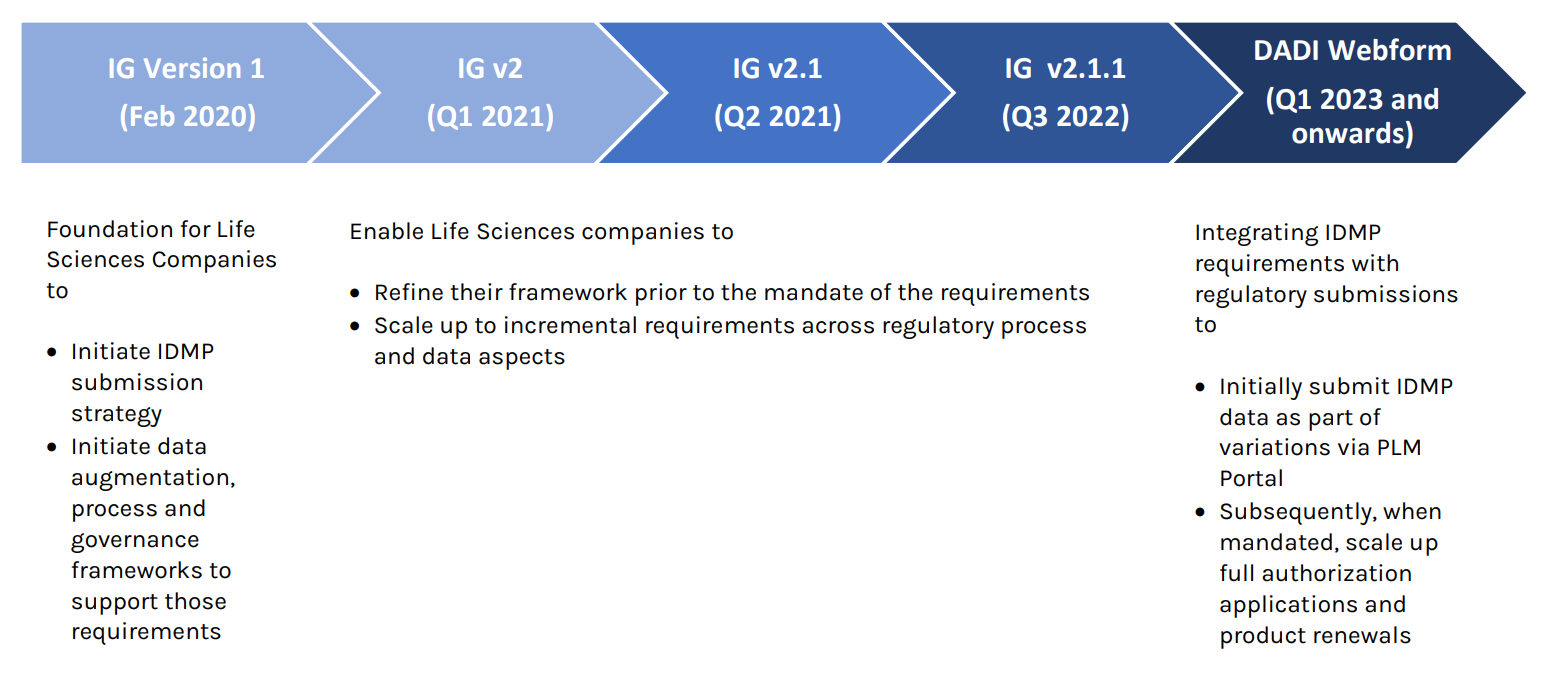
Implementation Guide Version 1.0 was published in February 2020 which provides a foundation for life science companies to build IDMP submission strategies, perform data augmentation, and establish process and governance frameworks that conform with its requirements. The publication of Implementation Guide Version 2.0 provided life science companies with an opportunity to refine their Target Operating Model (TOM) prior to when the requirements go into effect. Life science companies will be obliged to submit IDMP data within the defined EMA messaging format in the future.
Currently, EMA has chosen to bring in IDMP through regulatory submissions of increasing scope. The Digital Application Dataset Integration (DADI) Network Project will replace current PDF-based electronic applications forms (eAFs) with new web-forms in a new PLM (Product Lifecycle Management) portal. During 2022 and 2023 the eAFs for variations and initial marketing authorization will be replaced for human and veterinary medicinal products. Furthermore, the renewals eAF will also be replaced.
In 2022, EMA is focusing on replacing the current pdf-based electronic applications forms by web-based forms as part of implementing the TOM. The first form to be replaced is the variation eAF. Forms supporting other regulatory procedures, such as the initial marketing authorization, will follow. The Product Management Service (PMS) is supporting implementation of the electronic forms by making ISO IDMP-compatible product data available on Centrally Authorized Products (CAPs) and non-Centrally Authorized Products (non-CAPs).
IDMP Attribute Collection
There are over 300 discrete attributes described in the IDMP standards. While DADI webform requires a small proportion from these data elements, the attributes required for a product will depend on the product lifecycle status as investigational and authorized medicinal products have differing data models. Data relationships between attributes, or cardinality, can also be one-to-one, one-to-many, or many-to-many requiring the flexibility to accommodate multiple references to a single data point where appropriate.
Sources for IDMP attributes often exist today in an “unstructured” state which means they do not conform to a data model and have no easily identifiable structured sources, such as text within a Summary of Product Characteristics (SmPC).
A case for this is country-specific drug substance, product, or authorization details where a process or governance structure has not been put in place for maintenance within an information management system. As a result, these details under the current state remain in an unstructured form and will require transformation to meet IDMP requirements.
Leveraging technologies with a process-driven approach and well-defined data governance strategy is critical for organizations to manage the attributes in scope and transform data into assets. When data is structured under a well-defined governance framework, it can be used consistently and effectively across an organization, transcending traditional silos and paving the way to eliminate manual handoffs through automation. Structured data can then be treated as a true asset, one that drives efficiencies between departments and enable synchronization to pre-defined data models and better analytics.
Establishing a Data Governance Framework
Building a data governance framework transforms how an organization manages information and perceives its value.
A data governance framework establishes the roles and responsibilities for establishing compliance, maintaining data integrity, and ensuring the proper use of data Standards throughout the data lifecycle. IDMP attributes can evolve as the product evolves and so maintenance and decision-making are critical activities performed under the data governance framework.
Within the governing body, organizations should look to identify data stewards and data owners to ensure data is maintained per defined standards across an organization’s system landscape. This governing body can monitor the input of data into the originating source system and ensure the correct proliferation of this data to other systems as required.
Systems that can be implemented to support the data assets should include data dictionaries to keep the definitions and rules of each data element, data hubs to gather and transform the data in multiple other systems, monitoring tools to identify outliers and data errors, and analytics tools to create robust reporting and look for new ways to use the collected data.
IDMP was written to provide a unique identification to an approved drug in a country or territory, a key benefit in the eyes of health authorities. Industry, on the other hand, tends to look at the data from when the active ingredient is discovered and then built up. When the data elements from IDMP are transformed appropriately, the real benefit can be realized in industry.
Build a data governance framework that suits the needs of the organization with a business driver that also addresses compliance requirements.
Global Implementation of IDMP
A data governance framework should have processes and tools developed to leverage the data assets throughout the organization and across the product.
With the European Union implementing IDMP in a phased approach more commonly known as SPOR (substance, product, organization and referential data), other countries will soon follow suit as many continue to advocate for the standard to be adopted globally. FDA is looking to bring in elements of IDMP through its PQ / CMC project which streamlines the submission of CMC data as structured data and reviewing it via a Knowledge-aided Assessment and Structured Application (KASA). PMDA on the other hand has participated in global initiatives for IDMP but has not declared a timeline for IDMP implementation.
Why Red Nucleus R&D
IDMP is complex, requiring detailed knowledge of not just the guidance but of the regulatory information management landscape holistically, as well as a comprehensive understanding of data governance and management as it pertains to life sciences. Red Nucleus R&D is comprised of a dynamic team of IDMP experts who possess the required skills and capabilities to support Life Science companies with IDMP readiness and excellence.
Red Nucleus R&D delivers unrivalled R&D capabilities for life sciences teams. Our crossfunctional expertise, process-driven approach, and proven methodology reduce costs, remove silos and increase compliance and efficiency. Our operational, strategic consulting, and technology services span across R&D with a focus on all regulatory specialties within and across the entire product life cycle, for both major and emerging markets.