
DirectusPRO
A unique label management, tracking, and reporting solution
The only comprehensive labeling system that addresses the market gap in providing a system that can effectively and efficiently enable a global end-to-end labeling process.

Drive Operational Excellence
Ensuring efficient information flow from source label (CCDS or other) to local labels and across all local labels
Stay Compliant
Ensuring consistent scientific, medical, and administrative information across all labels globally
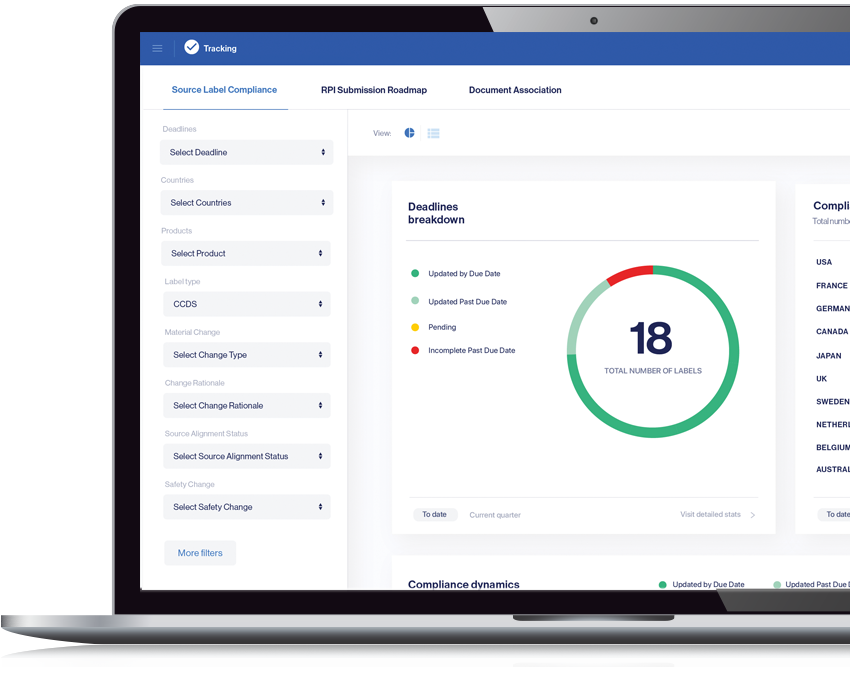
Streamline Tracking and Reporting
Ensuring traceability of historic changes globally at the content level (not only at the document level)
Powerful Benefits
- Label comparison and alignment with the ability to compare labels and manage content from version to version, or label to label
- Management of simultaneous labels in review ensuring compliance with the latest approved version
- Track and monitor label compliance from source label to local labels
- Component and collaborative based authoring with the ability to effectively manage content at the component level to drive efficiency, accuracy, and consistency across labels to ensure compliance
Key Features
- End to end label/document management from inception to dissemination, associated actions, and Health Authority interactions
- Component-based authoring with structured content for collaborative reuse
- Configurable workflows, metadata, document properties, and user security
- Simultaneous variations and versioning
- Document translation workflow
- Streamlined document comparison
- Structured Product Labeling (SPL)
- 21 CFR Part 11 compliant
We have been leading optimization and change in the end-to-end labeling process for the past 20 years. Now we're bringing together leaders like you with the Industry Labeling Advisory Committee Resource Center.