
A comprehensive mobile platform for decentralized research trials, evidence-based research, and learning programs.

We have a fully integrated and comprehensive platform that customizes a mobile health solution for Real World Disease Management, Decentralized Clinical Trial Management, and R&D Management.
Our fully integrated video capture capability is purposefully designed to capture critical endpoints in a clinical and at-home setting with the added video de-identification suite to enable regulatory submissions and review.
See iTakeControl in action
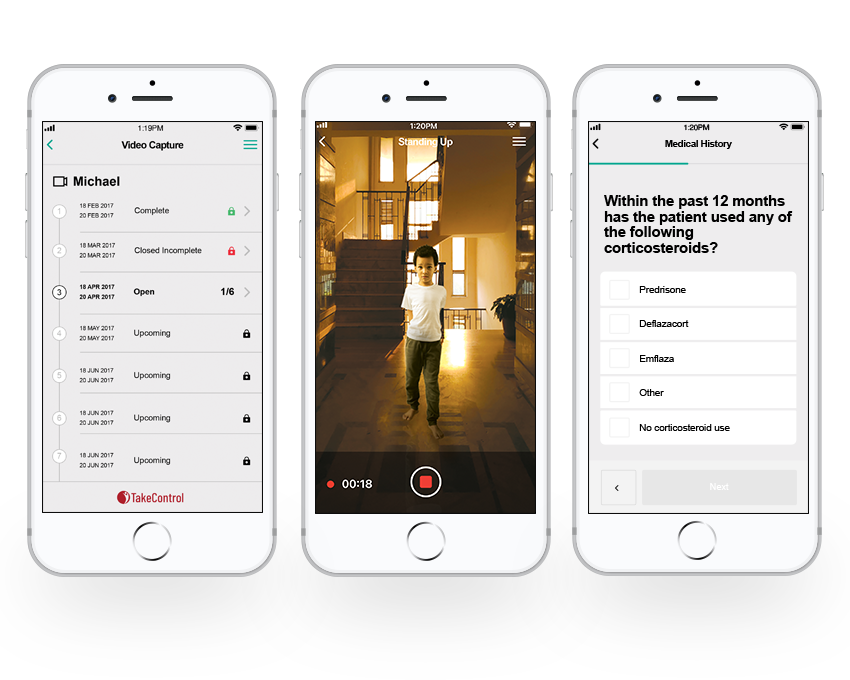
iTakeControl brings the power of video, telemedicine, ePRO, and eCOA together into a powerful, validated platform for consistent endpoints, quantifiable evidence, and remote clinical trial assessments, including clinical operational support and data management.
Mobile Platform
- Secure, encrypted, 21 CFR Part 11 compliant
- Full clinical video, vPRO & ePRO capabilities
- Customized study training
Study Management
- eConsent
- Full reporting portal
- Central Readers, Ratings, and Adjudication
Services
- Clinical operations
- Data management
- Registries and long-term follow-up
- Medical writing