Summary
Casimir, a CRO dedicated to better understanding disease progression and treatment benefit, has developed a novel outcome measure and endpoint for clinical research in patients diagnosed with Duchenne muscular dystrophy. The measure involves recording patients completing different functional assessments in a decentralized clinical trial model to demonstrate progression of a disease and change over time. Patients and caregivers needed to be trained in a real-time and compliant system that meets GCP requirements. Training involved guidance on how to complete the assessments, how to use the technology and platform, and how to be compliant for HIPPA requirements when submitting data for a clinical trial.
Situation Analysis
Training for patients has traditionally been to provide them with a PDF document that outlines the consent process. They are wordy, contain tremendous legalese that most patients are not familiar with, and are provided in a sterile hospital and often foreign environment. The use of real-world patients to create educational content and training provides engaging and efficient patient-focused training to meet the needs of patients and caregivers, while maintaining the requirements for global health authorities.
Evaluation
Training of patients and caregivers was initially developed using validation studies with the intended population to ensure proper understanding and effectiveness of training. Interviews were conducted with each participant in the validation study to gain feedback and to update training materials and instructions to ensure for the FDA the reliability of the training. Once validation studies are completed, the final training is uploaded to a mobile application that participants will use to collect and submit clinical data. A participant will log in and review training (including PDFs or videos) to ensure they know how to set up an assessment, how to conduct the assessment, and how to use the application to submit the required information. Once videos are submitted by a participant, the videos are reviewed by a data monitor to ensure the assessment was conducted appropriately and followed the steps and requirements outlined in the training. After review, each successful submission is approved and forwarded for scoring. If any issues with the data are determined, the data monitor can send a message to the participant to request additional review of training or provide additional learning/training requests or content and enable the participant to re-record or re-submit data based on updated training. This ensures that training materials are understood, tasks are performed according to requirements, and compliant data can be submitted and used in an FDA or other health authority application.
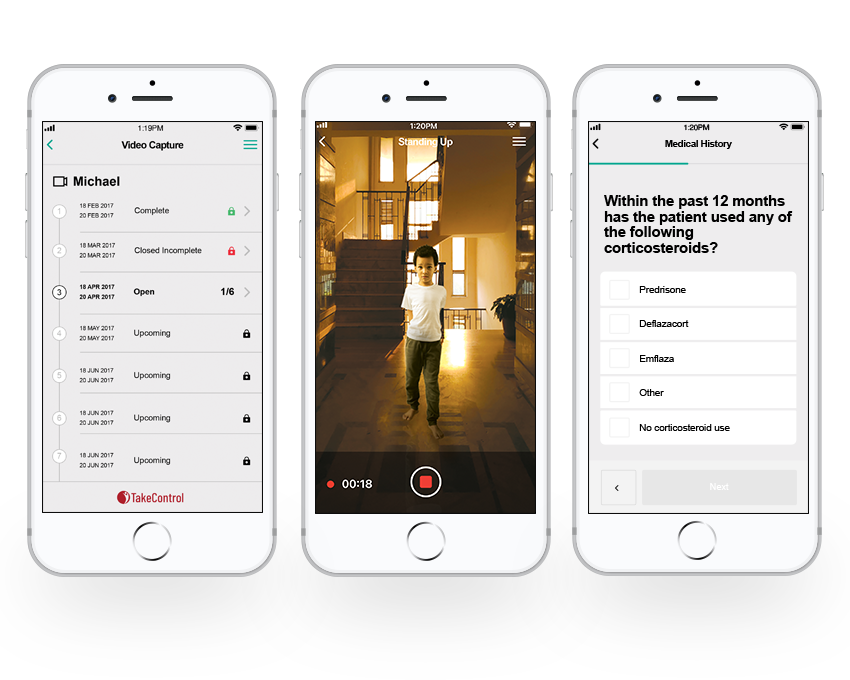
Methods/Approach
Casimir developed video training using patients and caregivers who had previously participated to educate clinical site coordinators, researchers, study staff, and stakeholders, as well as patients and caregivers for participation in a truly unique clinical study. The training was delivered to patients via the itakecontrol platform prior to any activities and electronically signed off to ensure compliance and consistency in the patient’s study participation.
Conclusions
Clinical research is highly regulated in the US by the FDA and by MHRA and EMA in the UK and EU, respectively. There are a number of standards and guidance that must be complied with for training. This project essentially allowed patients at home to be trained as investigators to enable real-time, at-home, real-world data to be assessed in the use of a treatment. Health authority requirements were met. Patients needed on-demand training to fit into their personal schedules to enable a patient-centric approach to data collection (vs a patient having to travel to a clinical site, sometimes hours away, and perform their “best” on demand). The training met the needs of the sponsor to capture standardized data, enabling efficient analysis of the outcomes to support evaluation of the medication for treatment.
Red Nucleus provides seamless, complete support for your clinical research needs. Our team of subject matter experts helps connect patients, clinicians, and sponsors through our integrated suite of products through clinical video assessments. We are here to support your day-to-day essential clinical activities in full compliance with health authority regulations.